October 24th, 1977
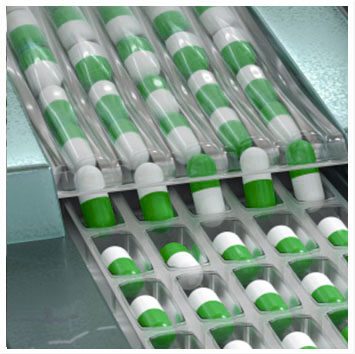
Under Decision No. 1176/BYT-QD dated October 24th, 1977, of the Ministry of Health, OPC Pharmaceutical Joint Stock Company was established through the merger of eight private pharmaceutical laboratories in former Saigon.

At the beginning of its establishment, No.26 Central Pharmaceutical Enterprise had a workforce of
![]() Just over 100 employees
Just over 100 employees
![]() The office and factory covered an area of approximately 7,000 square
The office and factory covered an area of approximately 7,000 square